Clinical Pipeline
Novel Approaches to Acute and Chronic Conditions
Edesa is exploring new ways to treat inflammatory and autoimmune diseases, including innovative alternatives to steroids, which can have serious side-effects.
Clinical Pipeline Status
Our current clinical studies are designed to further evaluate safety and efficacy. Since many of these drug candidates come to us with well characterized efficacy and safety data, we avoid much of the lengthy process of preclinical R&D studies and advance to meaningful data readouts more quickly.
Dermatological Diseases
Allergic Contact Dermatitis
Platform
Daniluromer – A First-in-class sPLA2 Inhibitor
Product Candidate
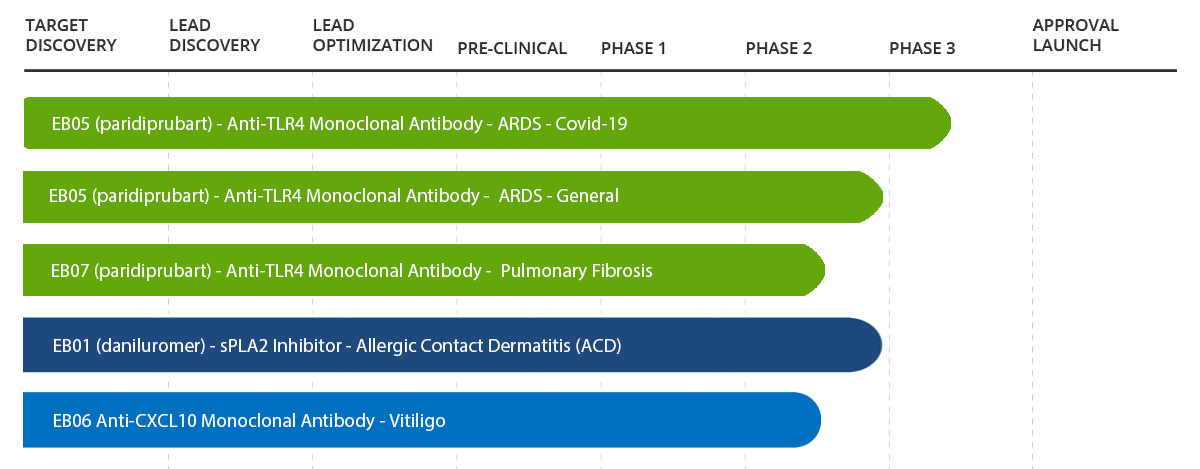
EB01 (daniluromer)
Product Information
EB01 is a novel sPLA2 inhibitor for the topical treatment of chronic Allergic Contact Dermatitis (ACD). EB01 employs a novel mechanism of action and in two clinical studies has demonstrated statistically significant improvement of multiple symptoms in contact dermatitis patients.
Disease State
Contact dermatitis is one of the most common occupational health illnesses in the United States, and has been estimated to cost approximately $2 billion annually. We estimate that there are more than 13.2 million people in the United States with contact dermatitis, with between 20% and 60% of all cases of contact dermatitis diagnosed as ACD. We believe EB01 will primarily benefit those who have chronic ACD.
Development Status
Edesa completed a Phase 2b double-blind, dose-ranging vehicle-controlled adaptive design clinical trial to evaluate the safety and efficacy of EB01 in a population with moderate to severe allergic contact dermatitis. See topline results.
Vitiligo
Platform
Immunotherapy – Monoclonal antibody
Product Candidates
EB06 Anti-CXCL10 Monoclonal antibody and
Immunotherapy – Monoclonal antibody
Disease State
Vitiligo is a life-altering autoimmune disease that results in the depigmenting of the skin. People who have vitiligo develop white patches of skin due to the destruction of special cells called melanocytes which produce the skin pigment melanin. The cause of vitiligo is not known but evidence strongly suggests that vitiligo is an autoimmune disorder in which the body’s immune system mistakenly targets and injures these cells. Vitiligo can affect any area of skin, but it commonly occurs on the face, neck and hands. According to the World Health Organization, vitiligo affects approximately 1% of the world’s population. It is a lifelong condition.
Despite high prevalence, vitiligo remains one of the most untouched areas in modern medical treatments and there has been little research compared to other immune disorders. Currently there are few treatment options available for patients with limited efficacy.
Development Status
Health Canada has approved our Clinical Trial Application for a Phase 2 study. See Press Release
Additional Dermatological Indications
Platform
sPLA2 Inhibitor and Others
Development Status
Edesa also intends to expand the utility of its sPLA2 inhibitor technology across multiple indications.
Respiratory Diseases
Acute Respiratory Distress Syndrome
Platform
Monoclonal antibody (mAb)
Product Candidate
EB05 (Paridiprubart)
Product Information
EB05 (Paridiprubart) is a first-in-class monoclonal antibody that has been engineered to alter inflammatory signaling by binding to and blocking the activation of TLR4
Disease State
Acute respiratory distress syndrome (ARDS) is a life-threatening form of respiratory failure, and the leading cause of death among COVID-19 patients. Prior to COVID-19, ARDS accounted for 10% of intensive care unit admissions, representing more than 3 million patients globally each year. ARDS has historically affected approximately 200,000 patients each year in the United States, resulting in nearly 75,000 deaths annually.
Development Status
Edesa is evaluating EB05 (Paridiprubart) as a potential treatment for hospitalized Covid-19 patients. Following promising Phase 2 results, the Phase 3 study design was granted Fast Track designation by FDA. Recruitment ongoing.
Compassionate Use
Please review our Expanded Access Policy.
Progressive Lung Fibrosis
Current Platform
Monoclonal antibody (mAb)
Product Candidate
EB07 (Paridiprubart)
Product Information
EB07 (Paridiprubart) binds specifically and selectively to Toll-like Receptor 4 (TLR4)
Disease State
Progressive lung diseases consist of a diverse group of interstitial lung diseases (ILD) characterized by accelerated respiratory failure, frequent disease exacerbation and earlier mortality. One of the most common types is idiopathic pulmonary fibrosis (IPF). The incidence and prevalence of IPF are increasing globally. This trend predates the pandemic. In addition, ILD is a common manifestation of systemic sclerosis and a leading cause of death.
Development Status
We are preparing an Investigational New Drug (IND) application.
Page Updated January 2024
sPLA2 Inhibitors
We believe that targeting the sPLA2 enzyme family with enzyme inhibitors will have a superior therapeutic effect because the inflammatory process will be inhibited at its inception rather than after inflammation has occurred.
TLR4 and CXCL10 Inhibitors
Countering Hyper-Immune Responses